Cold Chain Confidence: REGENXBIO’s Transport Validation Journey
read DetailsCold Chain Engineering

No matter how complex your cold chain challenge, Modality Solutions can craft a customized engineering solution that meets it head-on, bringing your therapy through a successful filing.
Request a Consultation
We apply our in-depth Cold Chain EngineeringTM expertise to solve the most difficult cold chain problems, focusing on the three areas that are vital to an effective supply chain: strategy, planning, and execution.
Modality Solutions has provided cold chain consulting for more approved therapies, qualified more thermal packaging solutions and participated in more regulatory interactions than any other engineering firm in the industry.
When you partner with us, you gain the benefit of the most experienced cold chain consultants in the industry—one with a proven track record of guiding the most innovative drug therapies to regulatory approval, efficiently and effectively.
Learn more about our specialized Cold Chain Engineering and Cold Chain Consulting capabilitiesNo matter where you are in the regulatory approval process, our Cold Chain Consulting Solutions will help you navigate your cold chain complexities and cross the finish line, quickly and effectively.
Leading pharmaceutical manufacturers, emerging biotech companies, and outsourcing partners rely on our cold chain consultants' expertise throughout their therapeutic’s journey—from NDA or BLA filing preparation, through the filing process, and after the product has reached the commercial market.
Are you unsure what cold chain risks your therapy will face?
Our cold chain consultants have the technical expertise to conduct a comprehensive risk assessment that ensures any safety, regulatory, or financial risks are identified and mitigated.
Do you need a fresh, third-party perspective on your cold chain?
Our cold chain experts can assess your current strategy, recommend the optimal design, and document our recommendation in a validation master plan.
Do you need to conduct transport simulation testing on your therapeutic?
We can conduct rigorous, comprehensive transport simulation testing of your drug product formulation against multiple hazards concurrently to confirm drug physical and chemical stability in transit.
Are you uncertain whether your shippers are equipped for the commercial supply chain?
Our thermal packaging experts can conduct a technical evaluation of the packaging used in your clinical trials and confirm if it’s scalable and cost-effective enough for the challenges of the commercial supply chain.
Do you need support for your regulatory filing?
Even if your network worked sufficiently during clinical trials, we can use the most current, proven validation techniques to prepare your therapy for commercial approval.
Are you in Phase I/II with your therapeutic?
Whether you’re moving through Fast Track, Breakthrough Designation, or Accelerated approval, we can help prepare your cold chain for regulatory scrutiny.
Are you in Phase II/III with your therapeutic?
We provide consultation on how to prepare your cold chain to meet the stringent regulatory requirements you’ll face in these review phases.
Have you received an Information Request?
We work fast to produce the robust data and reports regulators expect, heading off questions and avoiding prescriptive remedies that may not be ideal.
Is your product in the post-commercial phase?
As cold chain consultants, we can optimize your supply chain by recommending improvements, conduct best practices training, or provide the transport validation data you need for annual regulatory inspections

It takes a data-driven approach to validate your cold chain and ensure it withstands regulatory scrutiny and the rigors of the commercial supply chain. That’s what Modality Solutions provides through our unique Cold Chain Engineering capabilities. We’re the only provider that brings together every discipline needed for accurate, data-driven cold chain validation—combining the required chemical engineering, mechanical engineering, biomedical engineering, and packaging engineering expertise all under one roof.

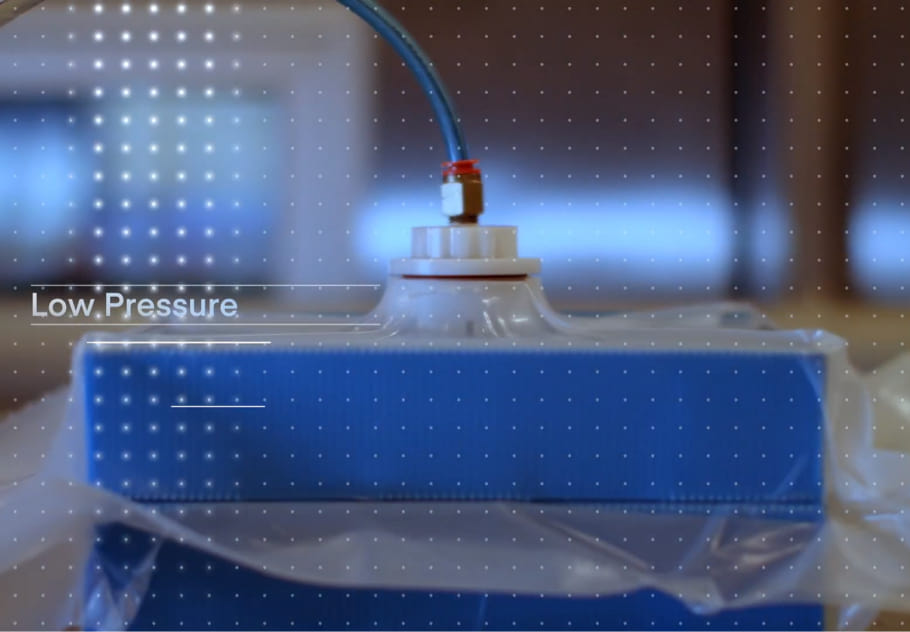
This enhanced plunger movement study approach helps you accurately demonstrate that your therapeutic in PFSs can withstand low pressure in transit and maintain both efficacy and sterility.
For drugs transported in pre-filled syringes (PFSs), it is vital to verify plunger movement in transit doesn’t impact the therapeutic’s sterility or efficacy, per ASTM standards D4169-16 and D6653-13. But traditional plunger movement studies have limited accuracy, often require large test quantities, aren’t suitable for some devices, or involve the use of substances (like lactose powder) that can impact later Container Closure Integrity (CCI) testing.
Modality Solutions developed a better way to conduct plunger movement studies—one that’s more accurate, requires fewer syringes, is suitable for use with any device type with a visible plunger, and reduces the use of lactose powder. Testing experts at our Transport Simulation Lab capture real-time video footage of plunger movement under low pressure, simulating transport by aircraft or by truck at high elevations, and use computer software to accurately measure the degree of movement.

Browse through our options based on your
therapy type or current clinical phase
Cold Chain Engineering

Ensuring the safe, effective delivery of small molecule drugs is more challenging than ever....
read DetailsAI-Driven

At a Glance: Cold Chain Engineering: Modality Solutions focuses on enhancing drug delivery through...
read DetailsCold Chain Engineering
