Cold Chain Confidence: REGENXBIO’s Transport Validation Journey
read DetailsCold Chain Engineering

As your controlled-temperature therapy advances through its development lifecycle, planning and optimizing the pharmaceutical cold chain is key to keeping moving towards approval. Modality Solutions has the regulatory experience and engineering expertise to support your therapy from early phase to post-commercial—with integrated cold chain solutions for every phase.
Request a Consultation
SERVICE TYPES BY PHASE |
|||
|
EARLY PHASE CLINICAL (PHASE I / II) |
LATE PHASE CLINICAL (PHASE II / III) |
FILING AND APPROVAL (READY TO FILE) |
COMMERCIAL |
|
Risk Assessment |
Transport Validation Master Planning |
Secondary Packaging Qualification |
Performance Qualification Reports |
|
Primary Packaging Qualification |
Drug Product Simulation Testing |
3PL Quality Agreements |
Cold Chain Optimization |
|
Logistics Network Design |
Thermal Packaging Qualification |
Performance Qualification Protocols |
|
|
Third-Party Logistics (3PL) Selection |
3PL GDP Audit |
Regulatory Filing Submission Support |
|
|
Formulation Optimization |
|||
|
Regulatory Support |
|||
|
Information Request Assistance |
|||
Planning for your therapy’s cold chain early and working with an experienced cold chain engineering partner helps you avoid costly delays in getting your drug product to market. Biopharmaceutical manufacturers often get a late start on addressing their cold chain requirements. Or they assume their in-house resources will be sufficient—only to find they don’t have deep regulatory and engineering expertise specific to their novel, first-of-its kind therapy
Preparing for your cold chain validation early in the clinical development process and working with the experts at Modality Solutions can keep both your development work and your regulatory filing on track. Our team brings the unmatched cold chain regulatory and engineering expertise it takes to bring new therapies to market. We’ve worked with 125+ biologics, advanced therapies, and drug-device combination products—including the industry’s most innovative advanced therapeutics—and engaged in hundreds of successful regulatory interactions.

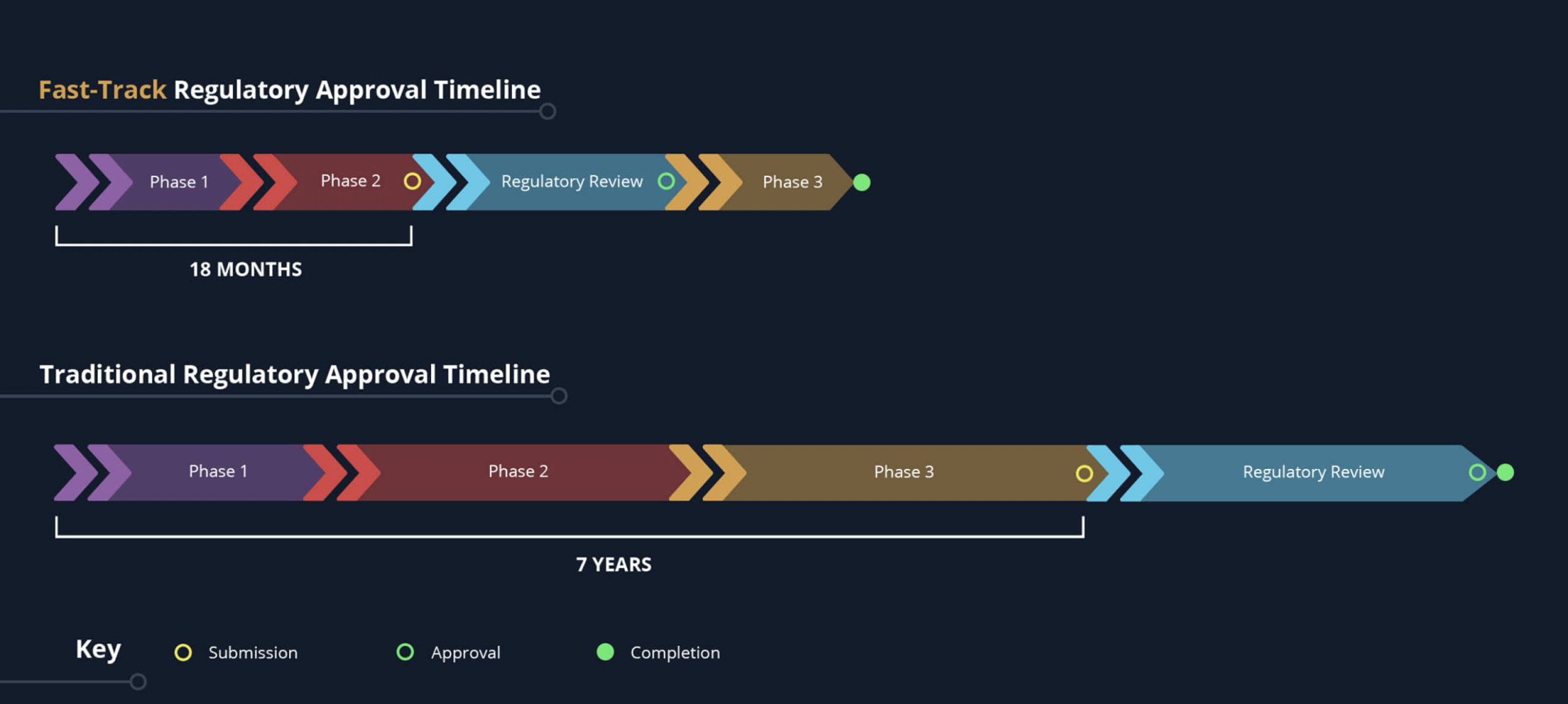
Whether your therapeutic is on an expedited approval pathway or a traditional approval timeline, we can provide a cold chain solution designed to get your drug product across the finish line successfully.

Cold Chain Engineering

At a Glance: Cold Chain Engineering: Modality Solutions focuses on enhancing drug delivery through...
read DetailsCold Chain Engineering

In the highly regulated and complex world of biopharmaceutical drug products, ensuring the integrity...
read DetailsBiologic
