Could your medical technology
benefit from our cold chain expertise?
Request consultation 
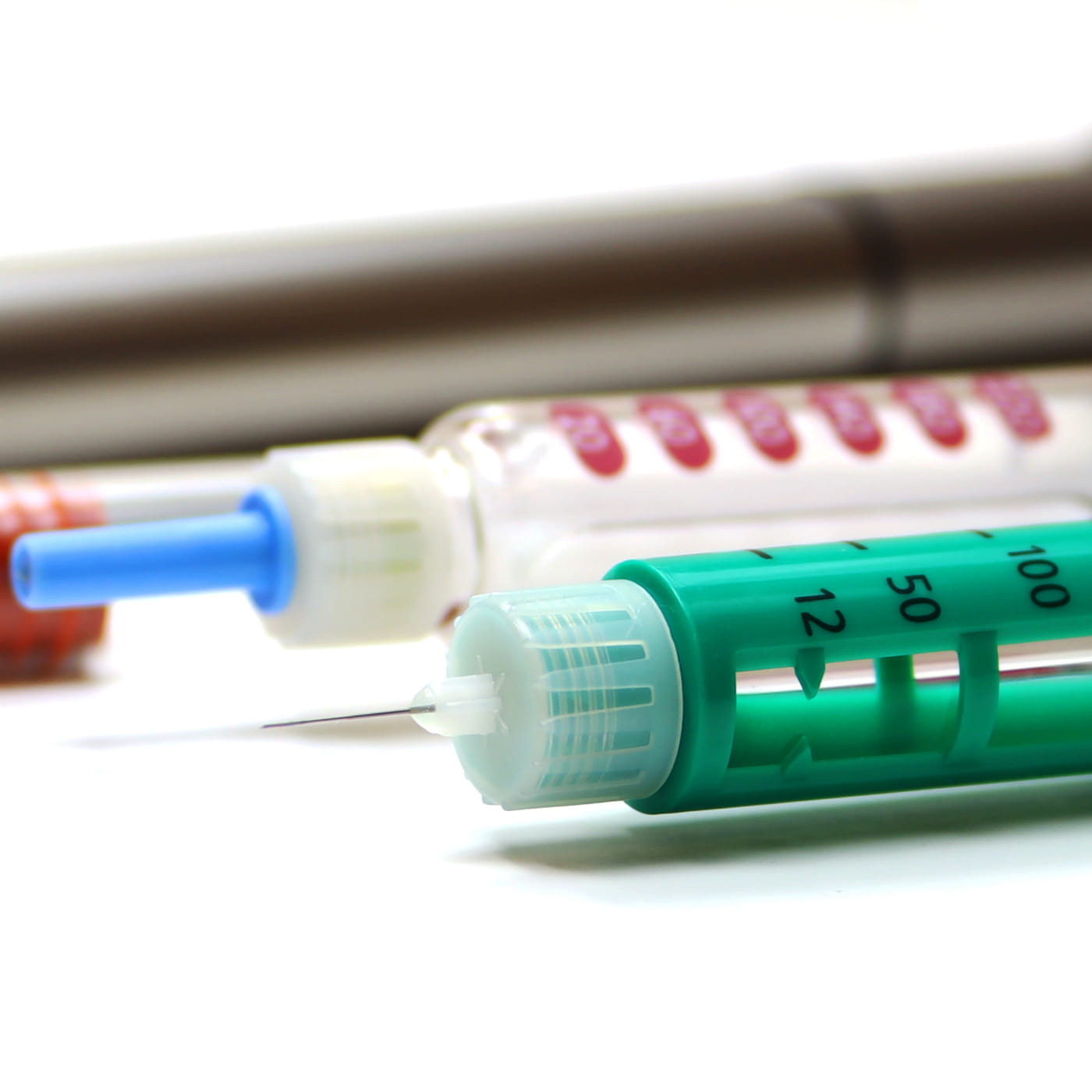
Helping MedTech innovators ensure their combination products are up to the challenges in the cold chain.
LET'S TALKMedical device, diagnostic, and digital health innovators are reshaping healthcare. Technologies like AI, robotics, patient-monitoring wearables, drug-eluting stents, and many others are helping people live longer and healthier. But transporting these breakthrough medical technologies is a challenging proposition with complex regulatory requirements.
When your MedTech is a combination drug/biologic-device therapy, bridging existing data to a new NDA or BLA filing means demonstrating your therapy will meet rigorous cold chain requirements. Whether you’re a biopharma looking to add your therapy to a device (like an autoinjector for drug delivery) or a device manufacturer looking to include a therapy on your device (like a drug-eluting stent), you must demonstrate that the device functionality and drug efficacy, purity, and potency aren’t impacted in the transportation process.
Modality Solutions’ transport validation studies and regulatory expertise help you meet rigorous FDA requirements for combination products and other innovative biomed devices. Our subject matter experts deliver everything you need to clear the hurdles and achieve a successful filing for your combination product or stand-alone medical technology.

ABBOTT VASCULAR CASE STUDY:
See how Modality Solutions identified temperature excursion reduction opportunities and minimized non-conformance rates for Abbott's Absorb stents.
View the case study now

We help prepare you for a successful regulatory filing every step of the way—assessing your cold chain, drug stability, packaging, and third-party logistics qualifications; developing a validation master plan; conducting transport simulation testing; and preparing your team for the CTD submission and pre-approval inspection.
Learn More
Whether you need turnkey support or you want to augment your in-house resources, our customizable Transport Simulation Solutions can deliver the robust data regulators expect during your drug’s review and approval. We assess transport risks, develop validation master plans, and rigorously test drug formulations and packaging for the five hazards that can occur in transport—concurrently and at the edges of the supply chain’s operating space—through our ISO-certified Advantage Transport Simulation Laboratory™.
Learn More
When you’re preparing for a new filing, responding to an Information Request, or looking to improve the supply chain for a commercial product, our Cold Chain Consulting and Cold Chain Engineering™ services are invaluable. Our cold chain consultants and experts draw on their extensive experience to help you overcome complex supply chain challenges, reduce risk, and meet regulatory requirements at every phase.
Learn More
Modality Solutions can conduct rigorous, comprehensive testing of your therapeutic against a wide range of ASTM and ISTA standards at our independent Advantage Transport Simulation Laboratory™ or a third-party lab—ranging from plunger movement studies to confirm sterility and efficacy, to drug-device functionality testing, and many other applications.
Learn More
We help enhance your cold chain’s performance for therapeutics that have already reached the commercial market—reducing shipping and packaging costs while ensuring your drug product remains protected in transit. From package design engineering and evaluation, to packaging selection support, to logistics network design, our Cold Chain Optimization Solutions ensure your supply chain is up to the challenge of transporting fragile or controlled-temperature therapies.
Learn More8.2%
The global market for drug-device combination products is forecast to grow at a compound annual growth rate of 8.2% through 2027.
77 M
The expanding geriatric population is expected to hit 77 million by 2034 in the US alone, spurring high demand for drug-device combination products.
Pervasive, chronic conditions like diabetes and cardiovascular disease will continue to drive demand for medical technology and BioMed devices.

Cold Chain Engineering
Learn More
Transport Simulation Lab
Learn More
AI-Driven Cold Chain Optimization
Learn More
Emergency Response to Regulatory Inquiries
Learn More
Today, four leading life science companies—Suttons Creek, Modality Solutions, CLINTREX, and ToxStrategies—announce their unification...
read DetailsBiologic

Ensuring the safe, effective delivery of small molecule drugs is more challenging than ever....
read DetailsAI-Driven

In the highly regulated and complex world of biopharmaceutical drug products, ensuring the integrity...
read DetailsBiologic
