No Temperature Controls Necessary For Small Molecules? Are You Sure?
Ensuring Quality for Small Molecule Therapy Supply Chains in a Growing Market Managing the...
read Details

The growing importance of refrigerated trailers in the pharmaceutical industry
Refrigerated trailers in the pharmaceutical industry are important now more than ever. In the United States, 14 million tons of pharmaceutical products are shipped by truck every year.1 In Europe, however, most pharmaceuticals travel by air or sea. Cold chain spending worldwide is estimated to account for $15 billion in 2018 out of a $182 billion biopharma logistics market.2 It is interesting to note that in the United States specifically, data indicates that most prescription products are handled two or three times before reaching its point of selling. It is imperative, therefore, for appropriate measures and precautions to be taken between each travel point, especially for refrigerated pharmaceuticals. The use and transport of refrigerated pharmaceuticals is on the rise, as it is projected that by 2022, 30 of the top 50 selling drugs will require refrigeration.3 The expanding need for refrigerated trailers has led to an increased need to run tests to compare temperature cycles.
What is the impact of the cycled setting?
Cold chain transportation in refrigerated trailers present a number of challenges in pharmaceutical supply chains. These challenges include inadequate packaging, delays, and most notably, interrupted temperature and climate control.4 Regarding this final obstacle, we have results to share. While running a series of controlled tests for a client, the temperature control unit (TCU) was inadvertently set to cycle mode, allowing us to compare and contrast the results of a “continuous” versus “cycled” setting.
Most if not all carriers of temperature sensitive pharmaceutical products have clear instructions to set the temperature control unit (TCU) to run in “continuous” operation. Why? The fan and compressor unit remain active and operating at all times to minimize the temperature differentials within the trailer. But, the TCUs have an alternate setting for fuel saving. In this mode, the fan and compressor will cycle off when the set point temperature is achieved. The TCU will “wake up” when the thermostat temperature reads 3 to 5 degrees from the set point.
The temperature of the air passing thru the supply vent of the trailer is an indirect measurement of the mode of operation of the TCU. In continuous mode the extreme in temperature variation is plus or minus 3 degrees of the set point. In cycle mode, the lag time to activate the TCU can result in temperature spreads of over 5 degrees from the set point.
There is additional variance in the cycle mode because the fan operation also contributes to the overall distribution of temperatures within the trailer. The chart below is an example of cycle mode for a CRT at a cold exposure. The temperature variations violate both sides of the CRT limits.
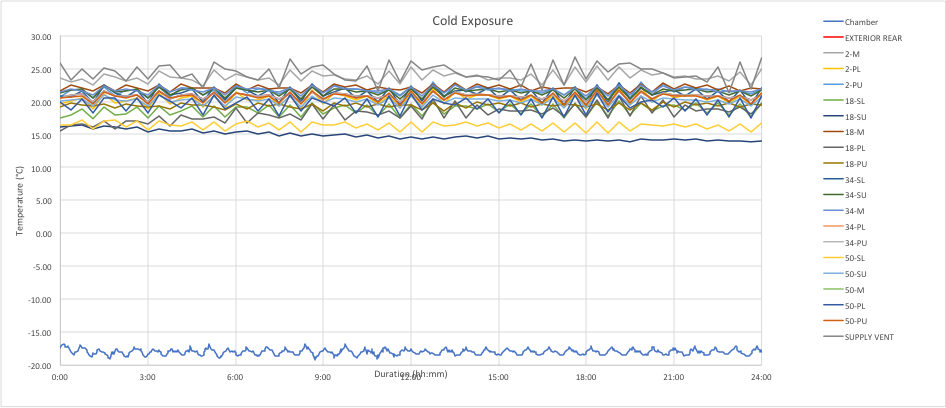
Clearly, the cycle mode will not meet the tight tolerance for refrigerated (5C) shipments. Controlled room temperature (CRT) shipments are also placed in jeopardy depending on the specific definition of CRT. Similar conclusions can be made about the cycle mode for the hot exposure. The results are demonstrated in the chart below.

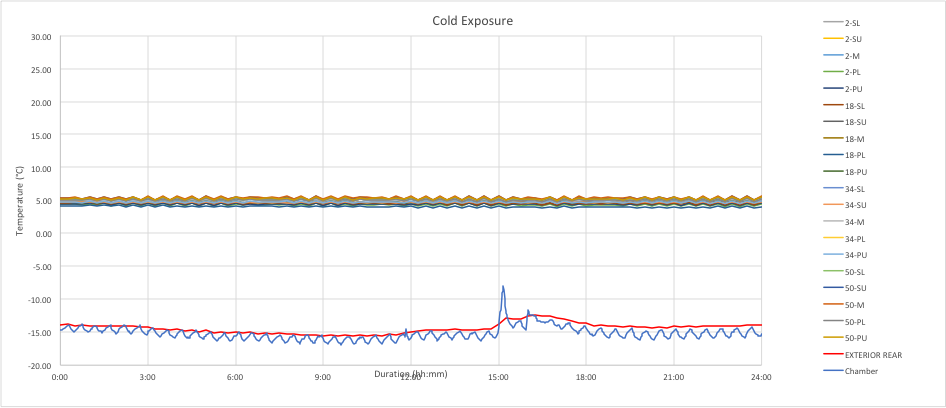
The chart below is an example of continuous mode for a refrigerated (5C) shipment at a cold exposure. The temperature variations are well within normal limits.
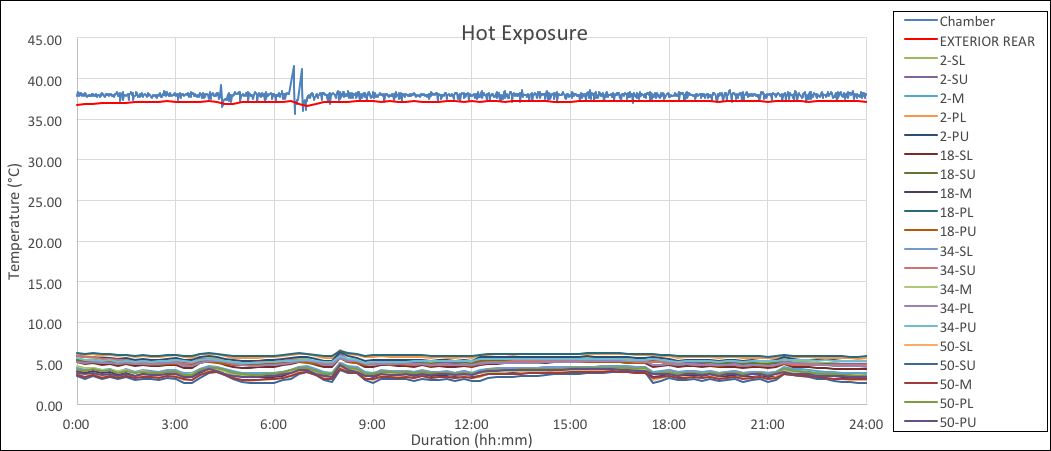
The chart below is an example of continuous mode for hot exposure. As seen by the graph the results were similar.
If the cycle mode is used at all, it should only be for frozen shipments when the setpoint is safely set well below the critical high temperature. The fuel savings between the two modes are less than $20 for a 24-hour period. These savings cannot justify the risk of wider trailer temperatures for either refrigerated or CRT pharmaceutical products.
Sources:
1. Basta, Lipowicz, 2018 Biopharma Cold Chain Sourcebook (Brooklyn: Healthcare Commerce Media Corp, 2018)., 51
2. Ibid, 63
3. Ibid, 23
4. https://unitedworldtransportation.com/4-common-cold-chain-logistics-problems-truckers-face-tips-solving/
Ensuring Quality for Small Molecule Therapy Supply Chains in a Growing Market Managing the...
read Details
Ensuring the safe, effective delivery of small molecule drugs is more challenging than ever....
read Details
At a Glance: Cold Chain Engineering: Modality Solutions focuses on enhancing drug delivery through...
read Details