No Temperature Controls Necessary For Small Molecules? Are You Sure?
Ensuring Quality for Small Molecule Therapy Supply Chains in a Growing Market Managing the...
read Details

You need a strategy to address the inevitable cold chain challenges caused by worldwide COVID-19 vaccine deliveries
Scientists are working round the clock to develop a COVID-19 vaccine. There are 23 vaccines in human clinical trials against the virus, SARS-CoV-2, according to the World Health Organization, with more set to begin testing soon.
“At the end of the day, you have to prioritize efficacy and then resolve the challenges around the logistics of the trial or logistics of manufacturing large quantities of vaccine,” said Pascal Soriot, AstraZeneca’s CEO, in a briefing with reporters.i
Developing a vaccine is only the first hurdle. Nearly 6 billion people will need an estimated two doses.ii One thing in common for most of the vaccine candidates that are the front runners in Phase 2 and 3 development are either genetic vaccines, viral vectored, or protein-based. As such, they will be highly unstable and susceptible to environmental hazards occurring during transportation. Ebola vaccine currently available is viral vectored and has to be stored below -60℃. If this is an indication of what is to come, the safe distribution of so many doses globally (see the chart below) will require a coordinated, robust, and reliable cold chain supply chain infrastructure that will take time to implement given the already existing supply chain shortages.
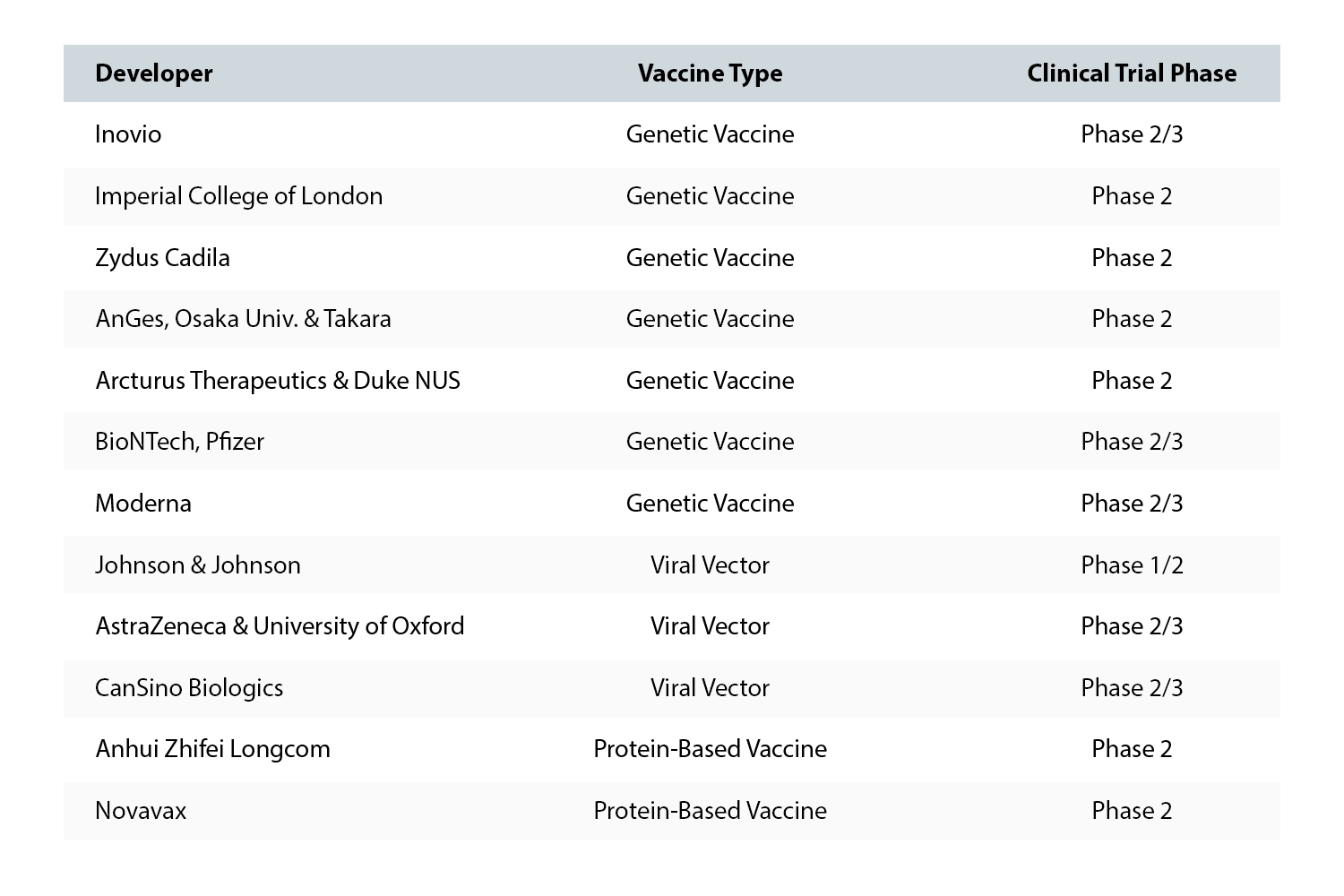
Stakeholders are to start designing their vaccine distribution cold chain immediately to guarantee timely and safe delivery of this much-coveted vaccine the entire world is anticipating.
Preventing failure entails first ensuring that raw ingredients maintain safe temperatures during transportation and storage. Then manufacturers must design a cold chain system that ensures the finished vaccine will withstand such environmental hazards as temperature fluctuations, shock, vibration, humidity, or pressure throughout storage, packaging, domestic and international shipping, distribution, and last-mile delivery.
There are many issues to consider, and some are unique to COVID-19. To minimize the risk that supply chain gaps may cause losses of valuable vaccines, two primary areas must be addressed:
The COVID-19 pandemic has caused shortages of ingredients and supplies. Sand used to manufacture glass vials is one example. Manufacturers must take extra care to minimize shipping damage not only to raw materials but also to supplies.
The pandemic is pressuring manufacturing capacity, too. Manufacturers are looking to design facilities that can pivot to other vaccines as some formulations fail. Their cold chains, therefore, must be flexible to accommodate changing requirements.
Most COVID-19 vaccine frontrunners use cell or gene therapy, which requires cryogenic temperatures during transportation and storageiii. The few protein-based formulations can use the conventional 2°C to 8°C) cold chain. Regardless of the vaccines’ exact temperature requirements, however, they all are highly sensitive to environmental hazards.
Simulation technology is a powerful tool vaccine developers can use to ensure the physical and chemical integrity of COVID-19 vaccines until it reaches the patient. Transport simulation can evaluate risks very early on and provide actionable data for mitigation. Whether this means using a qualified thermal shipper or adjusting the vaccine cold chain to achieve a better stability in-transit, the sooner you can prepare for safe door-to-door shipping, the sooner much-needed vaccines can be delivered to patients throughout the world.
Regulatory agencies have accepted simulation results for product and packaging validation. Unlike real-world testing, it lets organizations virtually test their supply chain strategy and cold chain processes under multiple conditions simultaneously, thus enhancing their supply chain’s resiliency and prepare for a successful regulatory approval in less time.
Early preparation is key. Evaluate existing resources and perform a risk assessment. Select and test thermal or cryogenic packaging, monitoring devices, and vials while vaccines are still being developed. This process enables packaging performance to be validated in conditions that match those of the proposed shipping lanes and transportation modes. Preparing early better ensures that packaging supplies are available when commercialization starts.
Simulation technology provides data that is a powerful time-saving tool for vaccine developers and an assurance of the physical and chemical integrity of COVID-19 vaccines. Contact Modality Solutions to learn how simulation testing can help you safeguard your cold chain.
Endnotes
i: Source:STATNEWS.COM
ii: https://www.supplychaindive.com/news/coronavirus-vaccine-supply-chain/579835/
iii: https://www.who.int/publications/m/item/draft-landscape-of-Covid-19-candidate-vaccines
Ensuring Quality for Small Molecule Therapy Supply Chains in a Growing Market Managing the...
read Details
Ensuring the safe, effective delivery of small molecule drugs is more challenging than ever....
read Details
At a Glance: Cold Chain Engineering: Modality Solutions focuses on enhancing drug delivery through...
read Details