More Than Cold Chain: How Modality Solutions Engineers a Full Spectrum of Supply Chain Success
At a Glance: Cold Chain Engineering: Modality Solutions focuses on enhancing drug delivery through...
read DetailsCold Chain Engineering

Find out how our Transport Simulation Lab can improve your drug stability testing.
Request a ConsultationYou need confidence that your drug formulation will maintain its stability throughout the supply chain. Given the time and expense it takes to bring a new therapeutic to market—and the increased regulatory scrutiny your advanced therapy will face—there’s no room for error. But real-world shipping tests don’t achieve worst-case conditions that your therapeutic may actually face in transit. That’s why Modality Solutions developed the Advantage Transport Simulation Laboratory™—the only multi-modal contract lab with the capability to test for all five transportation hazards, concurrently and at the edge of your cold chain’s operating space.




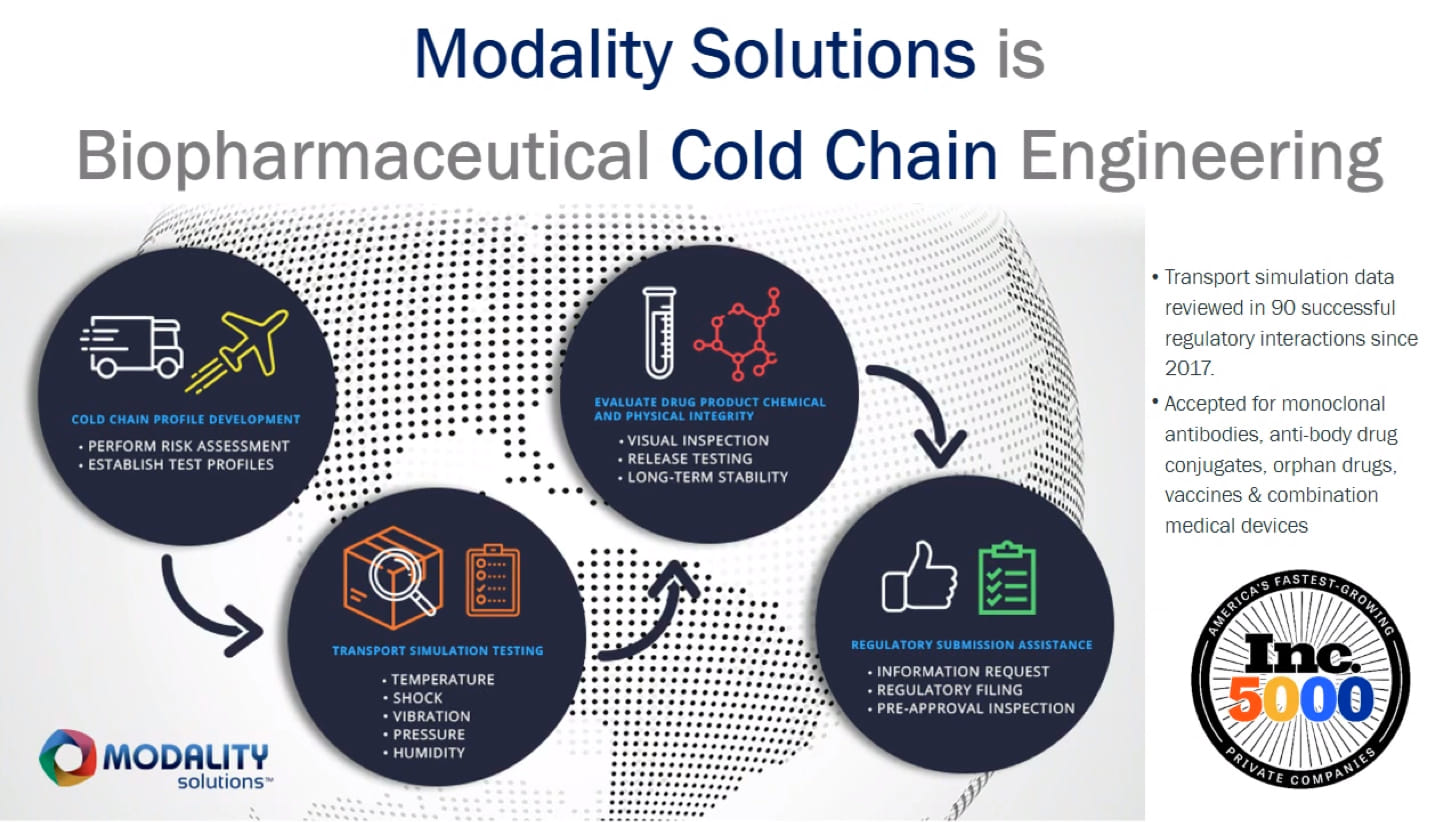
Transport simulation testing is now recognized industry-wide as the only way to accurately gauge how your therapeutic and your pharmaceutical cold chain will interact under worst-case conditions—and the only way to gain confidence that your drug will remain stable under those conditions. Our Advantage Transport Simulation Lab brings together the advanced facilities and engineering know-how to conduct rigorous transport simulation testing that yields the data you and your regulators need for assurance.
The Transport Simulation Lab concurrently tests for all five transportation hazards your drug may encounter in transit: temperature, pressure, humidity, shock, and vibration. Unlike sequential testing like the ASTM D4169 standard requires, or the snapshot-in-time that real-world shipping tests provide, our approach simulates the operating edges of your supply chain — yielding data that better reflects what may actually happen during transportation. Our engineers then translate that data into clear, usable information designed to give you and your regulators confidence in your drug formulation’s stability during transportation.
For protein-based drugs and other fragile therapeutics—like biologics, vaccines, monoclonal antibodies, antibody-drug conjugates (ADCs), cell and gene therapies, and any drug in pre-filledsyringes—the Transport Simulation Lab enables you to address regulators’ concerns about the stability of these therapy types in transit. And for Fast Track or other accelerated approvals, testing in our Transport Simulation Lab is typically the quickest way to obtain the robust data you need to address regulatory requirements and get your therapeutic across the finish line.

Our Advantage Transport Simulation Lab represents years of upfront engineering work combined with a commitment to ongoing innovations and enhancements that ensure our facility delivers the highest quality testing services.
Our lab operates under an ISO 9001:2015 certified quality system, ensuring adherence to rigorous and relevant testing standards. Equipped with full validation, our facility conducts precise and reliable transport simulation testing for accuracy and repeatability. We have the flexibility to test at the individual parcel level and the pallet level, at temperatures ranging from minus 35° Celsius to positive 60° Celsius, and at low pressures up to 20,000 feet in elevation. We conduct drop testing from various heights and orientations, test to any industry-standard profile, and use advanced equipment like our Kaye Validators and calibrated thermocouples to obtain highly precise temperature measurements.
When it comes to accurately simulating drug transportation risks, we know it’s critical that you get it right, quickly and efficiently. So we invest in continuous improvements that enhance our lab’s uptime, reliability, and performance. From custom vial boxes that better protect vials from breakage during shock testing, to a vibration table cage that enables secure stacked pallet testing, to a rigid vacuum chamber for conducting reliable plunger movement studies, we’re continually adding the most advanced equipment to deliver the best quality output for every transport simulation study. We also make constant improvements in our processes—fine-tuning SOPs, enhancing preventive maintenance activities, and enhancing how we store and back up your critical testing data.
It all adds up to a transport simulation lab that’s unmatched in its ability to provide the robust data you need to gain confidence in your therapy’s stability in transit.
Modality Solutions’ founders Gary Hutchinson and Dan Littlefield discuss the technical advantages of conducting transport simulation testing vs real-world shipping tests for drug formulation stability. This on-demand webinar reviews how to incorporate transport simulation into your regulatory filing, the technical limitations of real-world transport testing, what transport simulation can accomplish, and how it can help qualify your shipping lanes.
View the webinarOur Transport Simulation Lab is critical to obtaining robust, relevant data on your drug formulation’s stability in transit. But that’s just the start. Our wide range of technical capabilities and engineering solutions complement our lab testing, providing the comprehensive support you need to ensure your cold chain is optimized and your drug product is protected during transportation.
See which solutions are right for your therapeutic type and your current clinical phase.

With in-depth cold chain expertise and extensive technical engineering capabilities, Modality Solutions is uniquely qualified to help you successfully file and gain approval for your temperature-controlled therapeutics.

Cold Chain Engineering
Learn More
AI-Driven Cold Chain Optimization
Learn More
Emergency Response to Regulatory Inquiries
Learn MoreAt a Glance: Cold Chain Engineering: Modality Solutions focuses on enhancing drug delivery through...
read DetailsCold Chain Engineering

In the highly regulated and complex world of biopharmaceutical drug products, ensuring the integrity...
read DetailsBiologic

In the highly regulated and complex world of biopharmaceutical drug products, ensuring the integrity...
read DetailsBiologic
